For US Healthcare Providers
For US Healthcare Providers
FOR ADULTS WITH MODERATELY TO SEVERELY ACTIVE RA, IN COMBINATION WITH MTX
DEMONSTRATED SAFETY PROFILE
Adverse events (AEs) through Week 1121,2 :
| SIMPONI ARIA® + MTX | |
|---|---|
| Number of patients, n* | 584 |
| Median follow-up, weeks | 95.9 |
| Patients with ≥1 AE, % (n) | 79.1 (462) |
| Patients with ≥1 serious AE, % (n) | 18.2 (106) |
| Discontinuation rate due to AEs, % (n) | 7.0 (41) |
| Patients with ≥1 infection, % (n) | 49.1 (287) |
| Patients with ≥1 serious infection, % (n) | 6.2 (36) |
| Most common AEs (occurring in ≥5% of patients), % (n) | |
| Upper respiratory tract infection, 11.5 (67); bronchitis, 8.9 (52); RA, 8.7 (51); hypertension, 6.7 (39); nasopharyngitis, 6.7 (39); urinary tract infection, 6.5 (38); ALT increase, 6.5 (38); headache, 5.8 (34); pharyngitis, 5.8 (34) | |
*Patients may appear in more than 1 column.
Incidence per 100 patient-years for patients treated with SIMPONI ARIA® + MTX (n=584) in the controlled and uncontrolled phases1,2†:
- Active tuberculosis (TB): 0.28 (3 events; 95% CI [0.06, 0.81])
- Opportunistic infections: 0.37 (4 events; 95% CI [0.10, 0.95])
- Total malignancies: 0.47 (5 events; 95% CI [0.15, 1.09])
†Total patient-years of follow-up were 958 for active TB and opportunistic infections and 955 for total malignancies. During the controlled and uncontrolled phase, 6 malignancies were reported in 5 patients treated with SIMPONI ARIA® + MTX.
Infusion reactions reported in the controlled and uncontrolled phrases (≈Week 92)1:
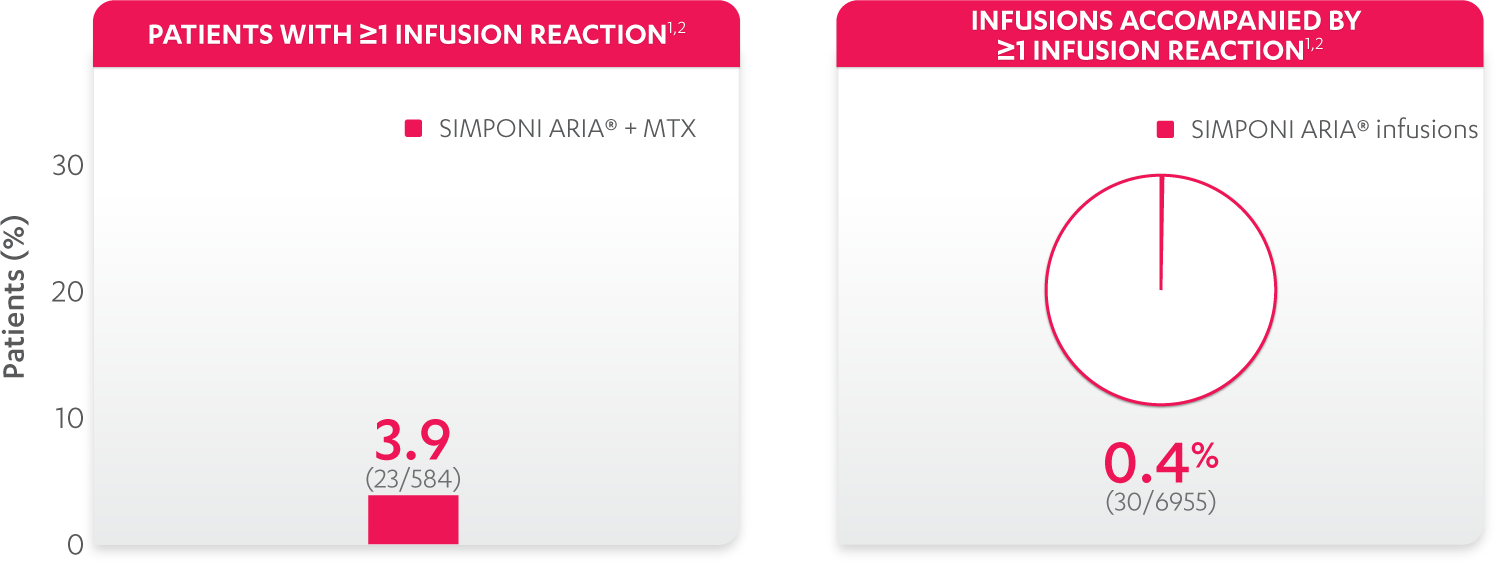
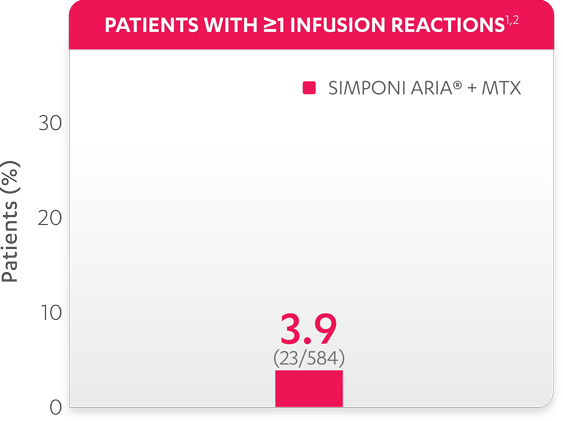
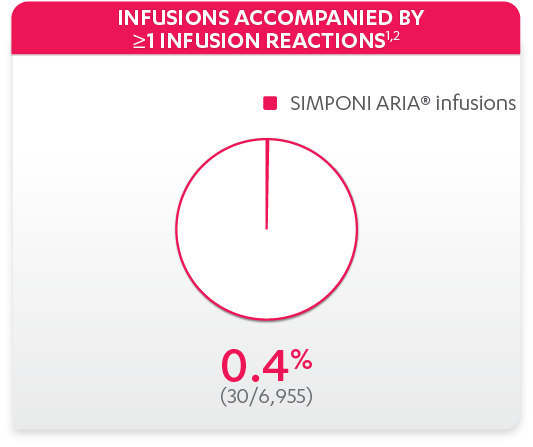
References: 1. Data on file. Janssen Biotech, Inc. 2. Bingham CO III, Mendelsohn AM, Kim L, et al. Arthritis Care Res (Hoboken). 2015;67(12):1627-1636.
