Choose A Condition:
Please Select
ACR20 response at Week 14 (primary endpoint): 59% of patients receiving SIMPONI ARIA® + MTX achieved ACR20 response vs 25% of patients receiving placebo + MTX (P<0.001)1,2
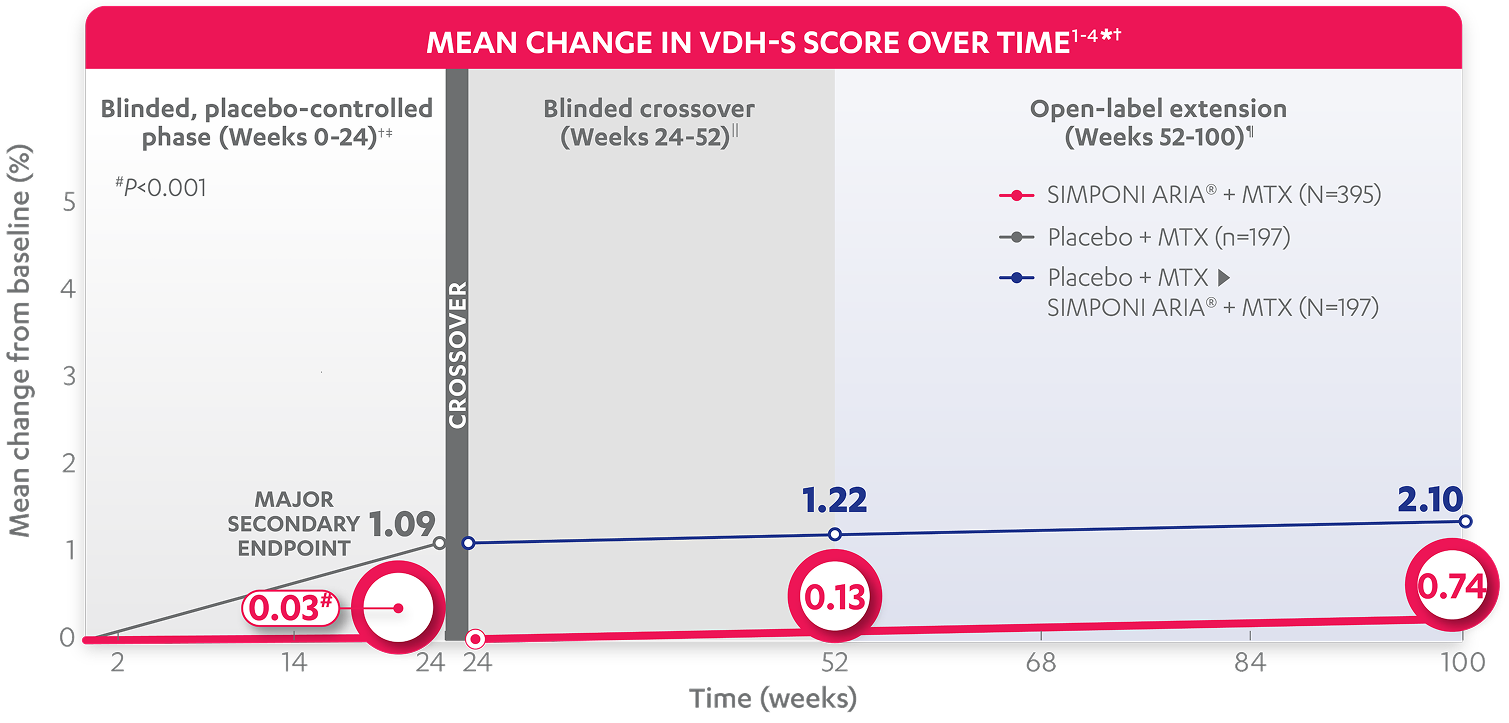
At Week 24: The mean change from baseline in total Sharp (vdH-S*) score was†
Blinded, placebo-controlled phase (Weeks 0-24)†‡
for patients receiving SIMPONI ARIA® + MTX (n=395) (P<0.001)
for patients receiving placebo + MTX (n=197)1,2
Calculated difference shows

inhibition of the progression of structural damage vs control group1§
MAJOR SECONDARY ENDPOINT

Calculated difference shows
Inhibition of the progression of structural damage vs control group1§
At Week 52¶: The mean change from baseline (%) Sharp (vdH-S*) score was
At Week 100: The mean change from baseline (%) Sharp (vdH-S*) score was
*The total modified vdH-S score (0-448) is a composite score of structural damage that measures the number and size of joint erosions and the degree of joint space narrowing in the hands and feet.
†The same patients may not have responded at each time point.
‡Missing data (linear extrapolation) rules were applied. For patients who early escaped, linear extrapolation was performed on the Week 16 data to the Week 24 endpoint.
§Percentage is the difference in mean total vdH-S scores divided by the placebo + MTX value.
||At Week 24, all remaining patients in the placebo + MTX group began receiving SIMPONI ARIA® + MTX in a blinded manner.
¶At Week 52, all sponsor personnel were unblinded to subject-level data.
Study design: GO-FURTHER™ was a global, multicenter, randomized, double-blind, placebo-controlled study in 592 adult patients who had moderately to severely active RA despite a stable dose of MTX (15-25 mg/week) for ≥3 months and who had not been previously treated with an anti-TNF agent. Moderately to severely active RA was defined as ≥6 swollen joints (out of 66 total) and ≥6 tender joints (out of 68 total), RF positive and/or anti-CCP antibody positive, and CRP ≥1.0 mg/dL. Patients were randomized to receive SIMPONI ARIA® 2 mg/kg + MTX (n=395) or placebo + MTX (n=197) as a 30-minute IV infusion at Weeks 0 and 4, and then q8w through Week 100. At Week 16, patients in the placebo + MTX group with <10% improvement from baseline in both swollen joint count and tender joint count began receiving SIMPONI ARIA® 2 mg/kg beginning with an induction regimen at Weeks 16 and 20, followed by maintenance infusions q8w in a blinded manner. At Week 24, all patients remaining in the placebo + MTX group began receiving SIMPONI ARIA® 2 mg/kg beginning with an induction regimen at Weeks 24 and 28, followed by maintenance infusions q8w in a blinded manner. All patients continued to receive MTX. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 14.2
ACR20=20% improvement in American College of Rheumatology criteria; CCP=cyclic citrullinated peptide; CRP=C-reactive protein; DMARD=disease-modifying anti-rheumatic drug; MTX=methotrexate; q8w=every 8 weeks; RA=rheumatoid arthritis; RF=rheumatoid factor; TNF=tumor necrosis factor; vdH-S=van der Heijde Modified Sharp score.
References: 1. SIMPONI ARIA® (golimumab) [Prescribing Information]. Horsham, PA: Johnson & Johnson. 2. Data on file. Johnson & Johnson. 3. Weinblatt ME, Bingham CO III, Mendelsohn AM, et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double-blind, placebo-controlled GO-FURTHER trial. Ann Rheum Dis. 2013;72:381-389. 4. Bingham CO III, Mendelsohn AM, Kim L, et al. Arthritis Care Res (Hoboken). 2015;67(12):1627-1636.