FOR ADULTS WITH ACTIVE PsA
DEMONSTRATED SAFETY PROFILE
Adverse events (AEs) reported in the placebo-controlled phase (Week 24)1-3:
| | SIMPONI ARIA® +/- MTX | | Placebo (saline) +/- MTX |
|---|
| Number of patients, n* | 240 | | 239 |
| Median follow-up, weeks | 23.9 | | 23.2 |
| Patients with ≥1 AE, % (n) | 46.3 (111) | | 40.6 (97) |
| Patients with ≥1 serious AE, % (n) | 2.9 (7) | | 3.3 (8) |
| Discontinuation rate due to AEs, % (n) | 2.1 (5) | | 1.3 (3) |
| Patients with ≥1 infection, % (n) | 18.8 (45) | | 15.5 (37) |
| Most common AEs (occurring in ≥5% of patients treated with SIMPONI ARIA® 2 mg/kg +/- MTX) |
| Alanine aminotransferase (ALT) increased | 7.9 (19) | | 2.1 (5) |
| Aspartate aminotransferase (AST) increased | 5.4 (13) | | 2.1 (5) |
- ALT elevations ≥5X the upper limit of normal occurred in 1.7% of SIMPONI ARIA®-treated patients and < 1% of placebo-treated patients1
- Adverse reactions were similar to those observed in patients with RA, with the exception of psoriasis (new onset or worsening, palmar/plantar, and pustular), which occurred in <1% of SIMPONI ARIA®-treated patients2
- The incidence of the adverse reactions reported in Trial PsA were similar to Trial RA with the exception of higher incidence in SIMPONI ARIA® for ALT increased (7.9% vs 2.1% in placebo), AST increased (5.4% vs 2.1% in placebo), and neutrophil count decreased (4.6% vs 2.1% in placebo)2
- No opportunistic infections or active tuberculosis were reported in the SIMPONI ARIA® or placebo groups1,2
- No malignancies were reported in the SIMPONI ARIA® group compared to 2 malignancies in the placebo group (n=2)1,2
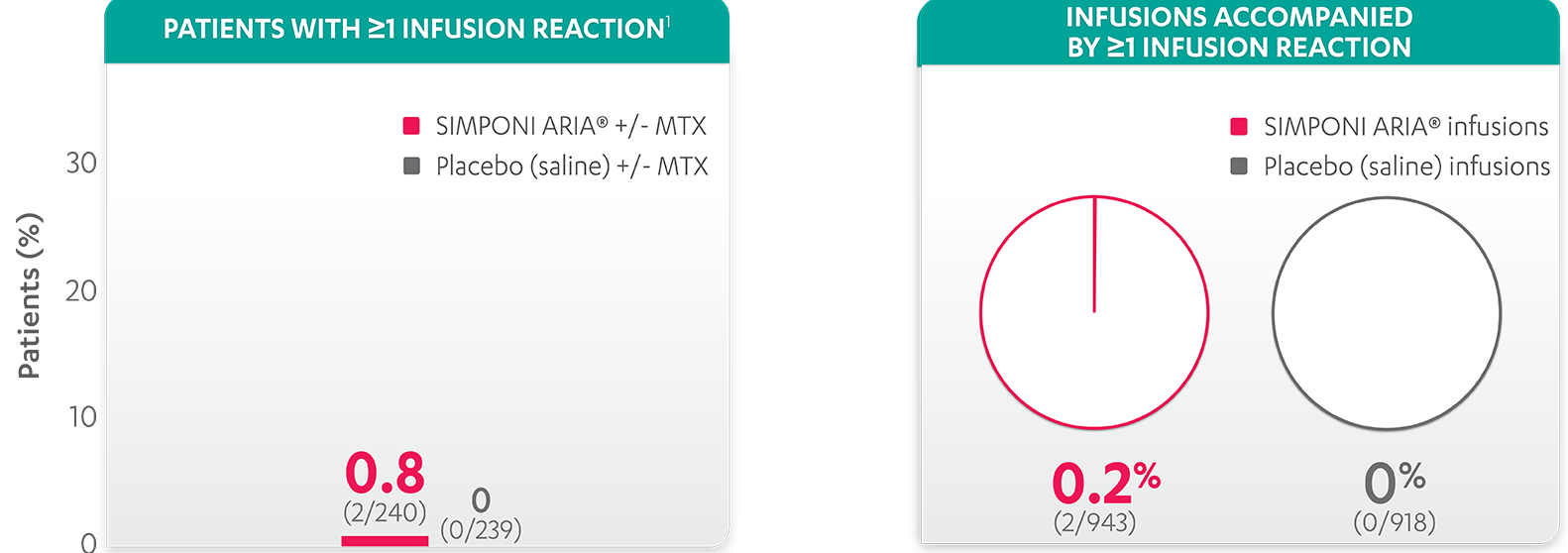
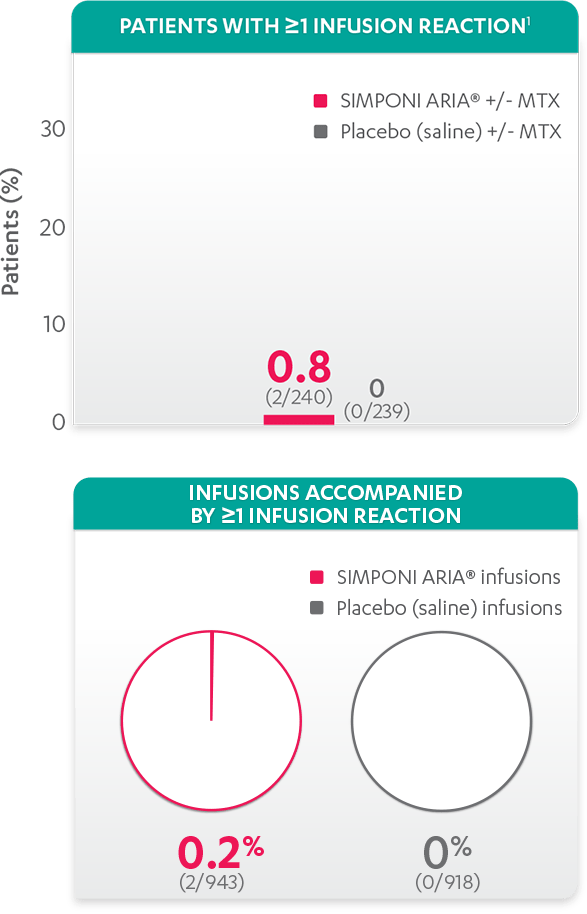
Infusion reactions reported in the placebo-controlled phase (Week 24)1:
- Infusion reactions reported for the SIMPONI ARIA® group included headache (1 patient, 0.4%) and infusion-related reaction (infusion reaction tightness of the chest, infusion reaction anxiety, and infusion reaction dyspnea; 1 patient, 0.4%)1
- None of these infusion reactions were considered severe or serious1
Back to Top >