Choose A Condition:
Please Select
ACR20 response at Week 14 (primary endpoint): 75% of patients receiving SIMPONI ARIA® +/- MTX achieved ACR20 response vs 22% of patients receiving placebo +/- MTX (P<0.001)1-3
Improvement in HAQ-DI score at Week 14 vs placebo: The mean improvement from baseline in HAQ-DI* score was 0.60 for patients receiving SIMPONI ARIA® +/- MTX (n=241) vs 0.13 for patients receiving placebo +/- MTX (n=239) (P<0.001)1-3
Through Week 14, patients receiving SIMPONI ARIA® achieved greater improvement from baseline in HAQ‑DI score vs placebo1†‡§
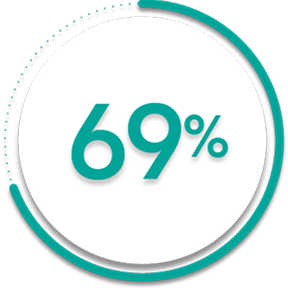
of patients receiving SIMPONI ARIA® +/- MTX (167/241)
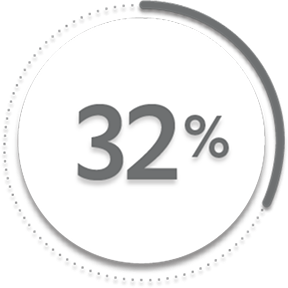
of patients receiving placebo +/- MTX (76/239)1
of patients receiving SIMPONI ARIA® +/- MTX (167/241)
of patients receiving placebo +/- MTX (79/239)1
Proportion of HAQ-DI responses were not adjusted for multiplicity. Therefore, statistical significance has not been established.
*A reduction in HAQ-DI score of ≥0.3 is clinically meaningful. The HAQ-DI is a validated questionnaire. It is scored from 0 (no disability) to 3 (completely disabled).
†The same patients may not have responded at each time point.
‡In this intention-to-treat analysis, patients were considered to be nonresponders if they experienced any of the following: increased the dose of MTX or oral corticosteroids over baseline for PsA not as part of early escape; initiated treatment with DMARDs, systemic immunosuppressives, treatment with oral, intravenous, or intramuscular corticosteroids, and/or biologics for PsA; met early escape criteria at Week 16 and started concomitant medication intervention; or discontinued treatment due to an unsatisfactory therapeutic effect. Treatment failure criteria were applied through Week 24 only. Last observation carried forward (LOCF) rules were applied to missing data.
§Change from baseline in HAQ-DI score is based on imputed data using LOCF for missing data.
References: 1. Data on file. Johnson & Johnson. 2. SIMPONI ARIA® (golimumab) [Prescribing Information]. Horsham, PA: Johnson & Johnson. 3. Kavanaugh A, Husni ME, Harrison DD, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty-four of the GO-VIBRANT study. Arthritis Rheumatol. 2017;69(11):2151-2161. doi: 10.1002/art.40226.