For US Healthcare Providers
For US Healthcare Providers
FOR ADULTS WITH ACTIVE PsA
SIMPONI ARIA® IS THE FIRST AND ONLY ANTI-TNFα BIOLOGIC WITH FACIT-F IN THE LABEL
ACR20 Response at Week 14 (primary endpoint): 75% of patients receiving SIMPONI ARIA® +/- MTX achieved ACR20 response vs 22% of patients receiving placebo +/- MTX (P<0.001)1-3
Treatment with SIMPONI-ARIA® resulted in improvement in fatigue as measured by FACIT-F2
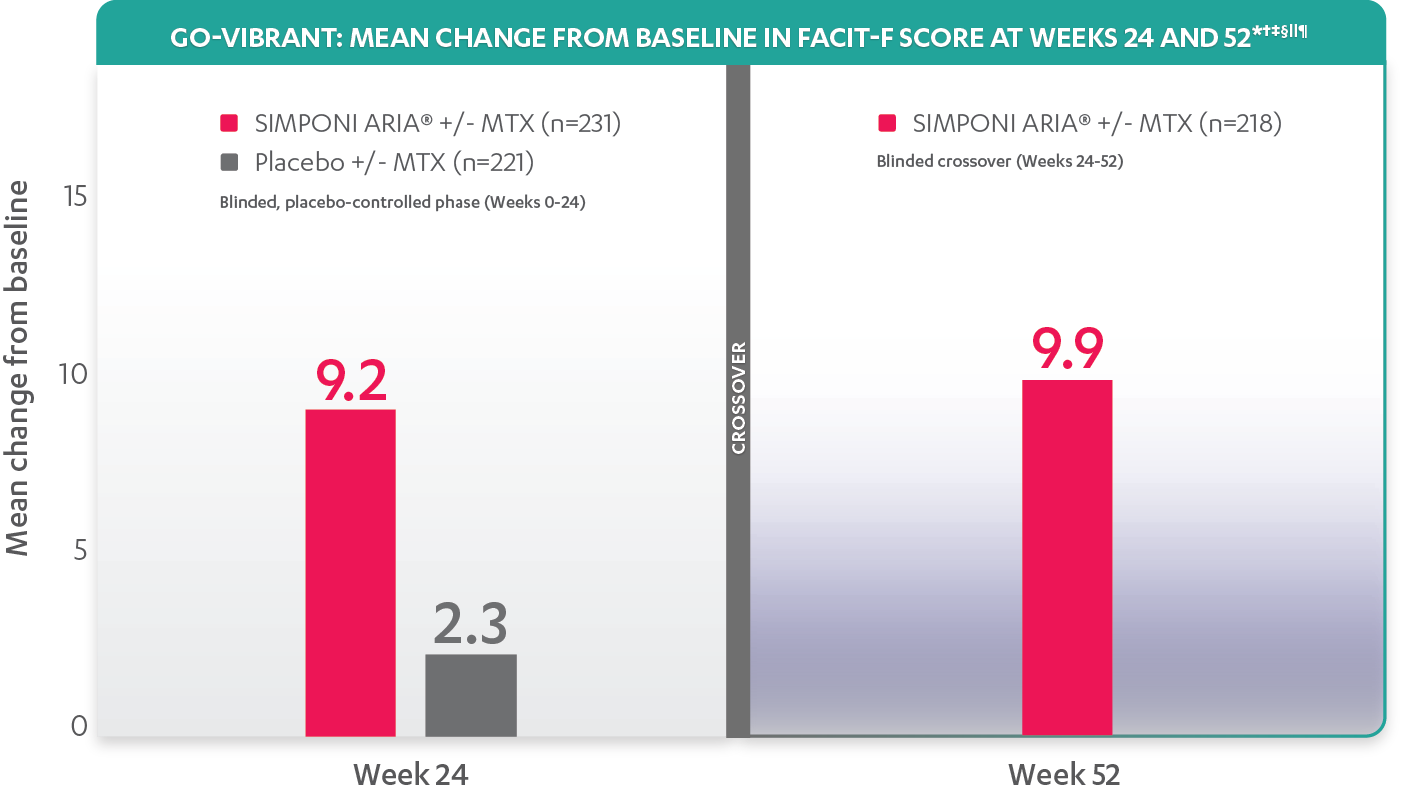
The FACIT-F endpoint was not adjusted for multiplicity. Therefore, statistical significance has not been established.
PATIENTS WITH A ≥4-POINT IMPROVEMENT IN FATIGUE AS MEASURED BY FACIT-F
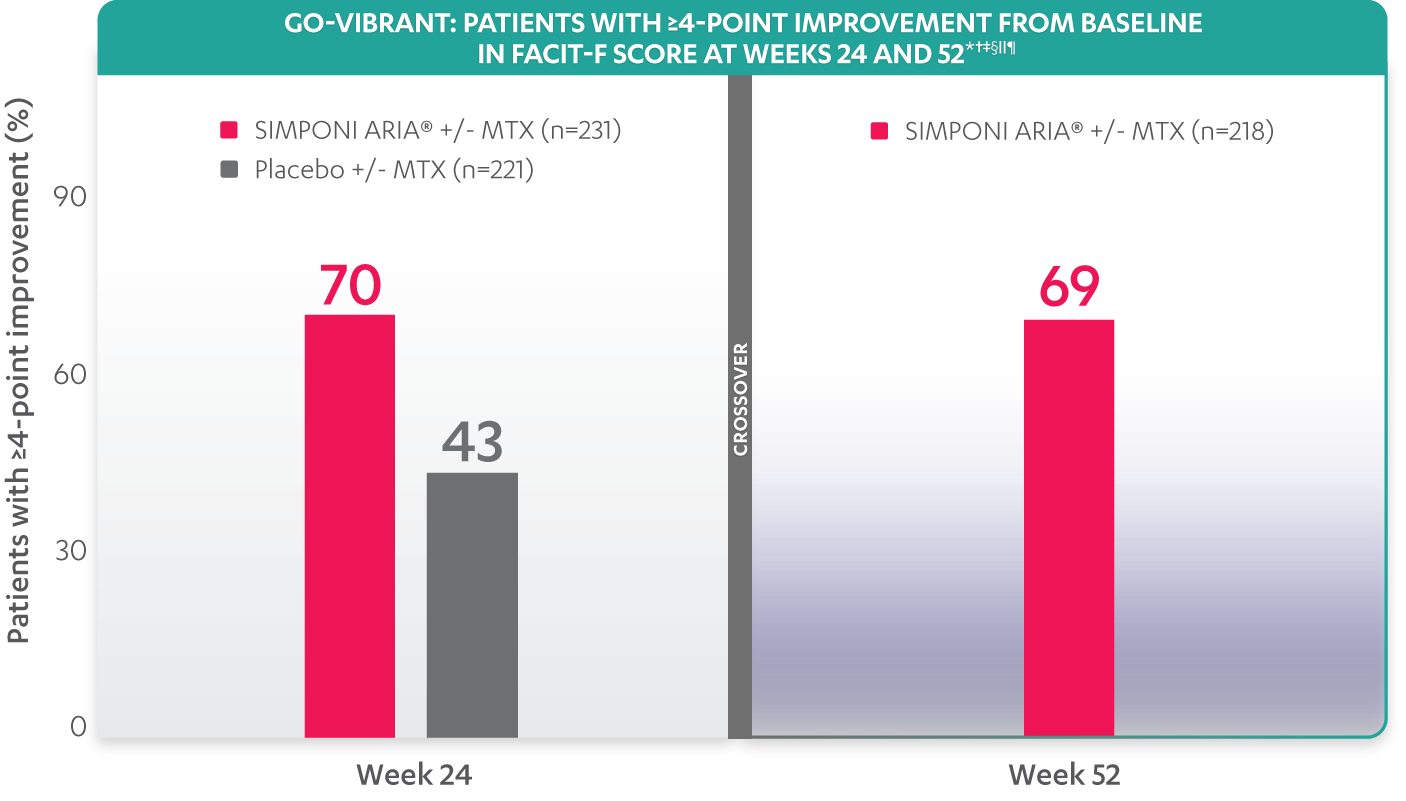
The FACIT-F endpoint was not adjusted for multiplicity. Therefore, statistical significance has not been established.
The threshold for clinically meaningful improvement when assessing fatigue using FACIT-F in clinical trials was a change of ≥4-point improvement.
*Change from baseline in FACIT-F score is based on observed values.
†In rheumatology, a change of 4 points in FACIT-F score was considered meaningful and has been used as response definition in the PsA population.
‡The same patients may not have responded at each time point.
§Although not developed for PsA, FACIT-Fatigue has been used to assess fatigue in rheumatology, and its properties have been evaluated for the PsA population.
||Improvement from baseline in FACIT-fatigue score is based on observed values.
¶After Week 24, patients and doctors knew that all patients were on SIMPONI ARIA® (blinded active treatment), which may have affected the results.

INFUSION EXPERIENCE
SIMPONI ARIA® offers an efficient 30-minute infusion.
DOSING CALCULATOR
Calculate an adult patient's dose of SIMPONI ARIA® based
on weight.
ACCESS AND AFFORDABILITY
View first-line coverage rates for SIMPONI ARIA®.

