For US Healthcare Providers
For US Healthcare Providers
FOR ADULTS WITH ACTIVE AS
DEMONSTRATED SAFETY PROFILE
Adverse events (AEs) reported in the placebo-controlled phase (Week 16)1,2 :
| SIMPONI ARIA® | Placebo (saline) | ||
|---|---|---|---|
| Number of patients, n* | 105 | 103 | |
| Median follow-up, weeks | 16.1 | 16.0 | |
| Patients with ≥1 AE, % (n) | 32.4 (34) | 23.3 (24) | |
| Patients with ≥1 serious AE, % (n) | 1.9 (2) | 0 | |
| Discontinuation rate due to AEs, % (n) | 0 | 0 | |
| Patients with ≥1 infection, % (n) | 11.4 (12) | 7.8 (8) | |
| Most common AEs (occurring in ≥5% of patients treated with SIMPONI ARIA® 2 mg/kg) | |||
| Nasopharyngitis | 5.7 (6) | 1.0 (0) | |
*Patients may appear in more than 1 column.
- The adverse reactions were similar to those reported in patients with RA, with the exception of the higher incidence of ALT increased, which occurred in 2.9% of SIMPONI ARIA®-treated patients compared with none of the placebo-treated patients
- No opportunistic infections, active tuberculosis, or malignancies were reported in the SIMPONI ARIA® and placebo groups1,2
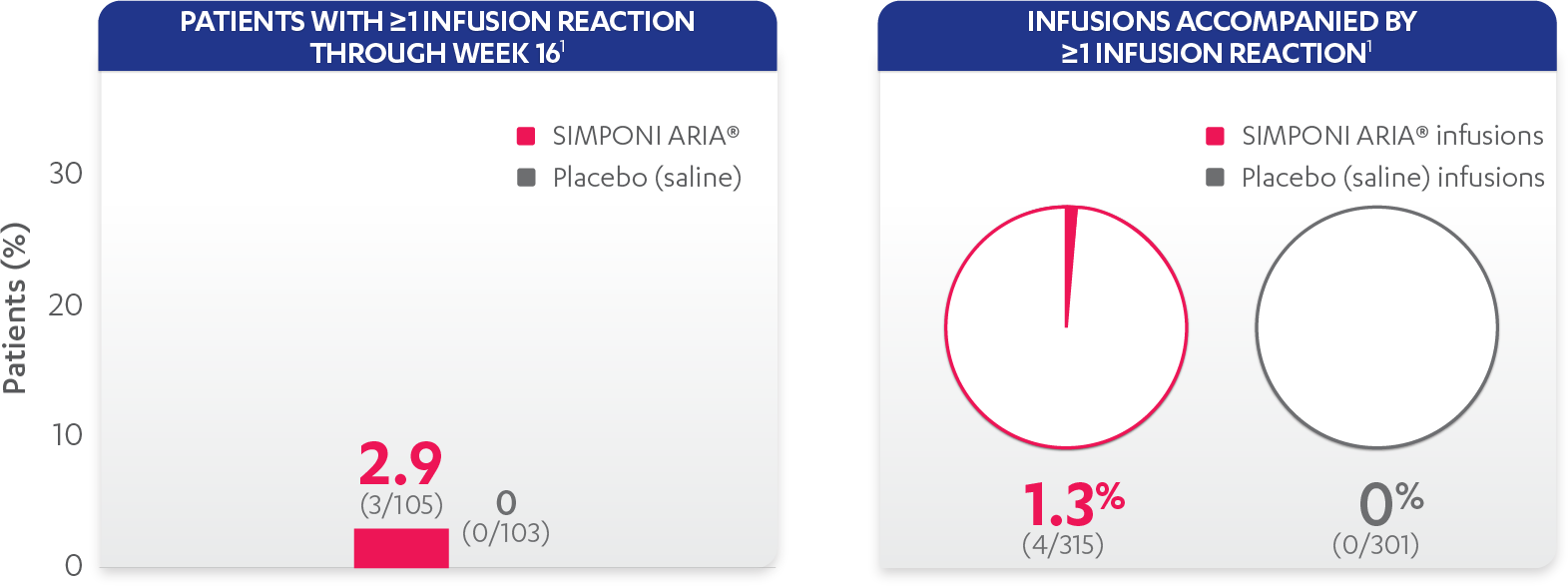
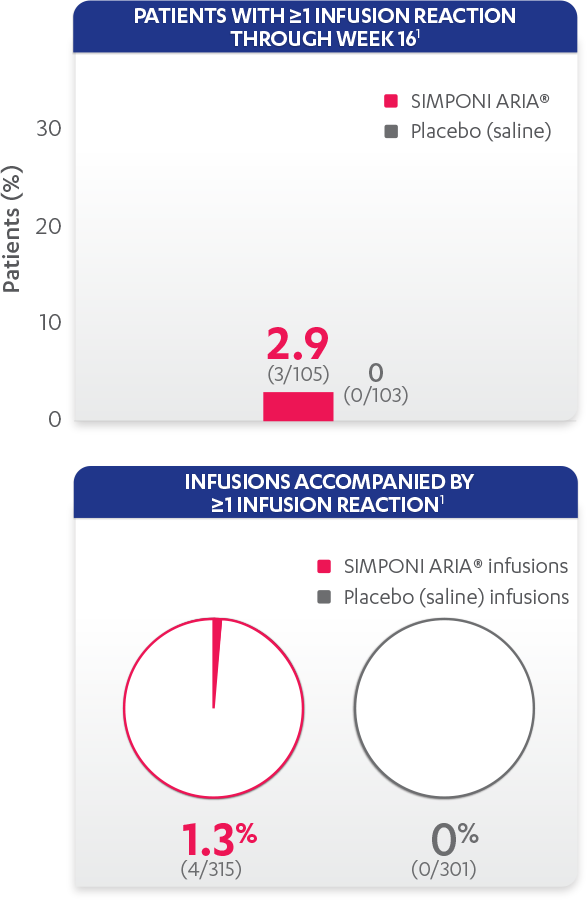
Infusion reactions reported in the placebo-controlled phase (Week 16):