For US Healthcare Providers
For US Healthcare Providers
FOR ADULTS WITH ACTIVE AS
REDUCTION IN SYMPTOMS OF ACTIVE DISEASE
ASAS20 response at Week 16 (primary endpoint): 73% of patients receiving SIMPONI ARIA® achieved ASAS20 response vs 26% of patients receiving placebo (P<0.001)1,2
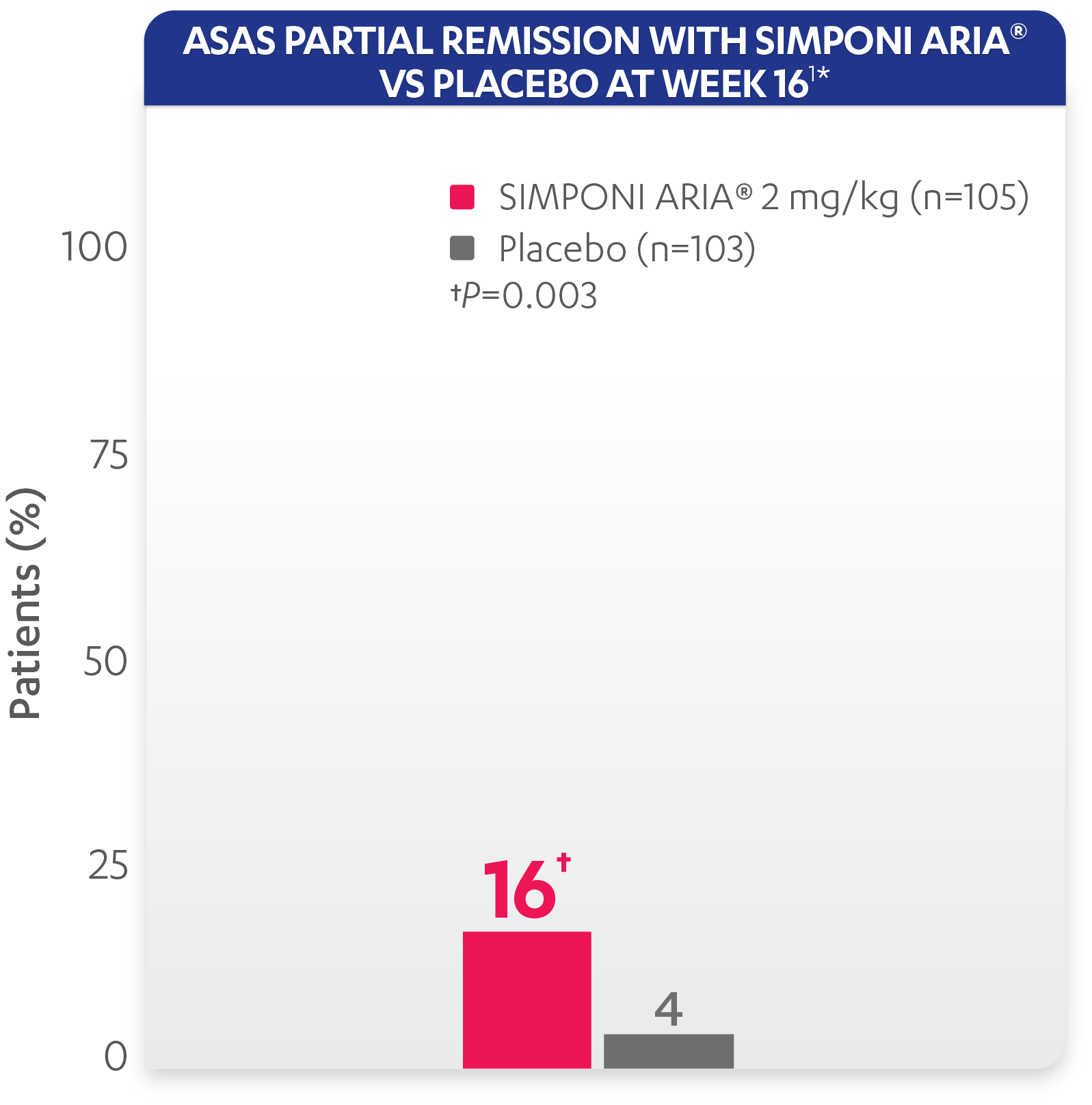
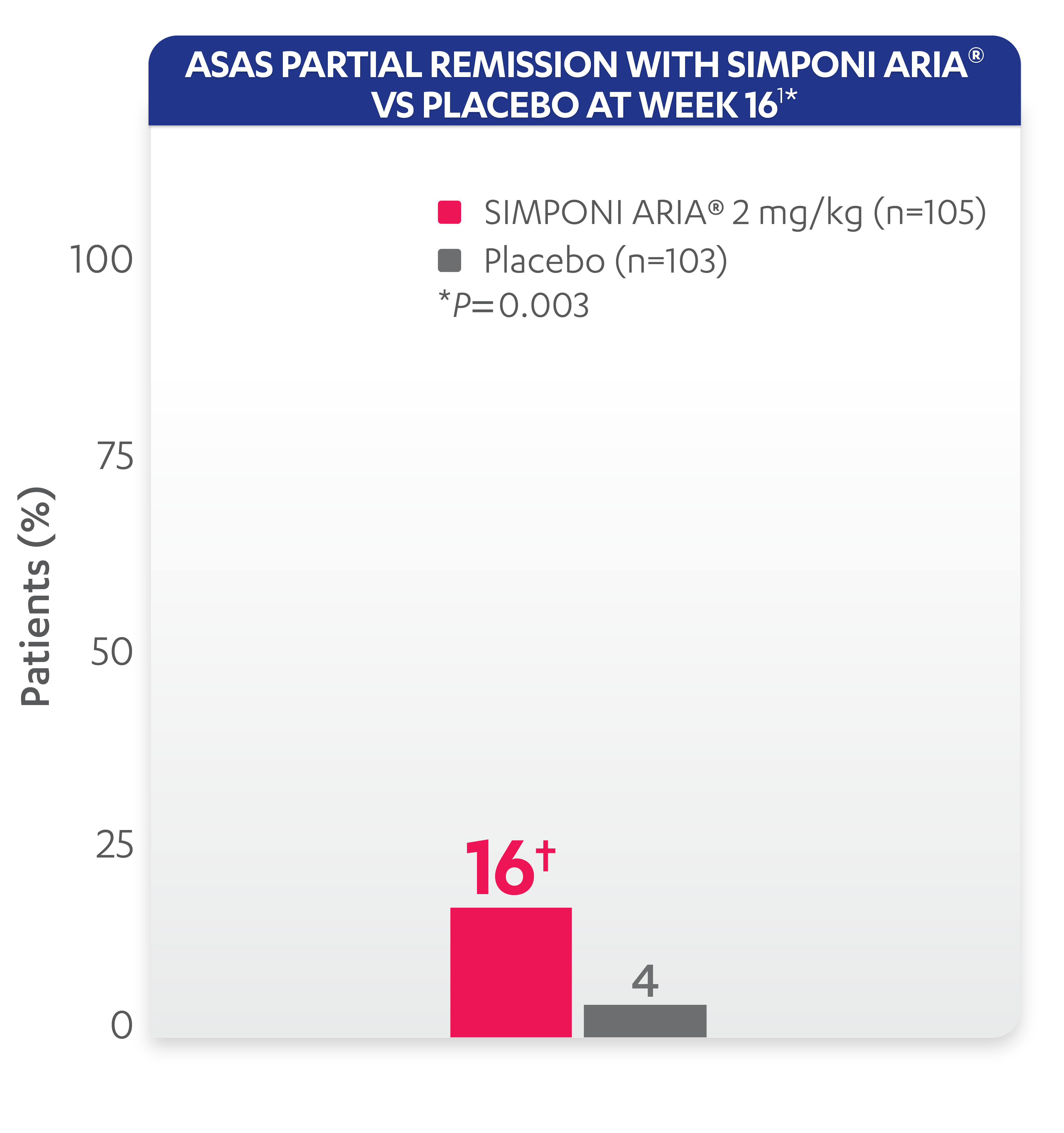
ASAS partial remission: Low level of disease activity was measured by criteria for “ASAS partial remission,” defined as a value below 2 on a scale of 0 to 10 in each of the 4 ASAS domains (patient global, total back pain, function [BASFI], and inflammation [average of the last 2 questions of the BASDAI concerning morning stiffness]).
*ASAS partial remission is based on imputed data using treatment failure, LOCF for partially missing data, and NRI for completely missing data.
Study design: GO-ALIVE was a global, multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of SIMPONI ARIA® compared with placebo in 208 adult patients with active AS with an inadequate response or intolerance to NSAIDs. Patients had a diagnosis of definite AS for at least 3 months according to modified New York criteria. Patients had symptoms of active disease [Bath AS Disease Activity Index (BASDAI) ≥4, VAS for total back pain of ≥4, on scales of 0 to 10 cm (0 to 100 mm), and a hsCRP level of ≥0.3 mg/dL (3 mg/L)]. At Week 0, patients were randomized in a 1:1 ratio to 1 of 2 treatment groups. Subjects in the placebo group (n=103) were randomized to receive IV placebo infusions at Weeks 0, 4, and 12. At Week 16, these patients were crossed over to SIMPONI ARIA® and received administrations at Weeks 16, 20, and q8w thereafter through Week 52. Patients in the SIMPONI ARIA® group (n=105) were randomized to receive SIMPONI ARIA® 2 mg/kg infusions at Weeks 0, 4, and 12. These patients received a placebo infusion at Week 16 to maintain the treatment blind and continued to receive SIMPONI ARIA® infusions at Week 20 and q8w thereafter through Week 52. Patients were allowed to continue stable doses of concomitant MTX, SSZ, hydroxychloroquine (HCQ), low dose oral corticosteroids (equivalent 25 to ≤10 mg of prednisone per day), and/or NSAIDs during the trial. The primary endpoint was the percentage of patients achieving ASAS20 response at Week 16.